Saliva Test for COVID-19
The Solution
Background on the Current Situation with Testing
There are three primary types of tests being deployed during this pandemic: RT-PCR, rapid antibody, and rapid antigen. The PCR is a molecular or nucleic acid test, which actually determines if SARS-CoV-2, the coronavirus that causes COVID-19, is present. The specimen is obtained by deep nasopharyngeal swab. The antibody test looks for antibodies to the coronavirus in a fingerstick of blood. Since these antibodies only appear in the blood a number of days following the infection, it is only useful to know if some has had the disease and might now have some immunity. It is of no use for diagnosis. The antigen test also provides results quickly, but it has an extremely high percentage of false negatives. Thus, anyone who tests negative needs to then get an PCR test.
The PCR test is highly accurate, since amplification of the DNA enables it to pick up even small amounts of the coronavirus. It is the gold standard. But there are drawbacks. The nasopharyngeal swabbing is very unpleasant to endure. Plus, it’s dependent upon the person inserting the swab to go deep enough. Since the dynamics of that process are challenging, PCR tests have some false negatives as well. A small percentage, but still a bit concerning. Plus, the RNA extraction and other requisite steps at the lab has resulted in reporting of several days up to two weeks. Given the suboptimal situation with these conventional tests, there is a pressing need for a test that is easy to administer, close to 100% accurate, and with results within about 24 hours.


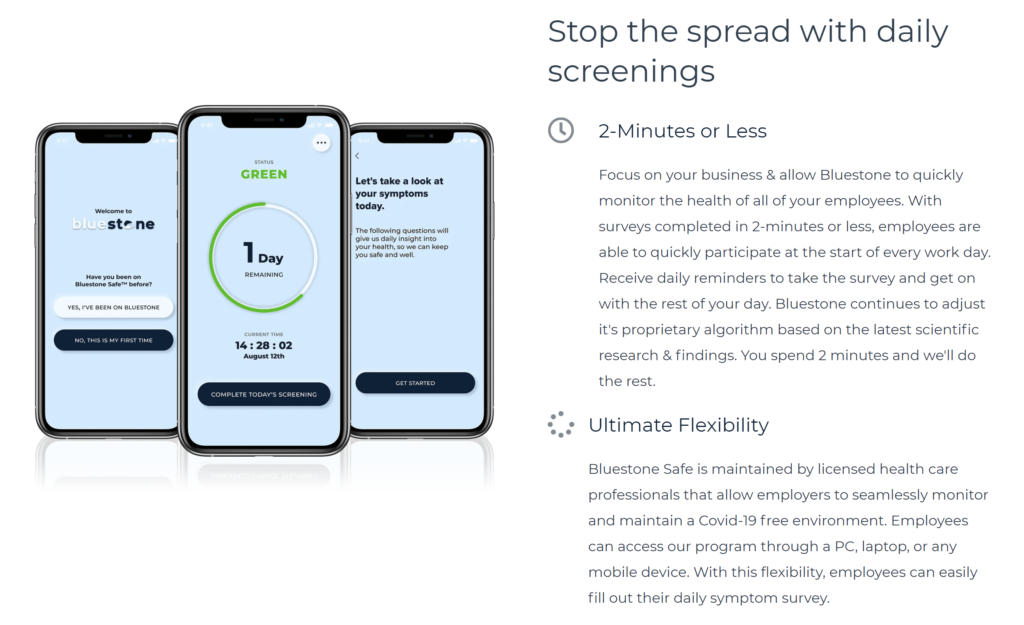
ABI teams up with bluestone USA to offers FDA authorized saliva test for covid19 the Bluestone Safe(TM) Saliva Test. It meets all the key requirements for the test should be universally used for COVID-19 diagnosis: easy, palatable, safe to administer, highly accurate, and fast results reporting. It has been in successful use for many months during the pandemic, and the support system is impressively elegant. Of particular note, there is no need for a PPE-garbed medical tech; people just spit in a tube that has a barcode on it. They enter that number into the Health Portal, to link to their electronic record. The tubes are overnighted to the lab, and the results will be available by the end of the next day. It just doesn’t get any easier than that. Also, since the saliva sample is not dependent upon getting enough specimen on a swab — and the sample is still put through the gold-standard PCR system — this test has been shown to be even more accurate than the swabbing approach. Let’s make testing all that it can be!
Evidence on the Accuracy of Saliva Testing
Here are a couple of excerpts from this article in the LA Times:
“The saliva tests did a better job of detecting the virus formally known as SARS-CoV-2, the researchers found. In the first five days after diagnosis, 81% of the saliva tests came back positive, compared with 71% of the nasopharyngeal tests. A similar gap remained through the 10 day after diagnosis. In addition, the researchers detected more copies of the virus’ genetic material in patients’ saliva than in the samples taken from the back of their nasal cavities.”
“To see how the tests stacked up among people with asymptomatic infections, the researchers recruited 495 healthcare workers with no signs of COVID-19 and gave them the saliva test. Thirteen of the tests came back positive. Among those 13 people, nine had given themselves nasal swabs on the same day, and only two of those tests came back positive. However, all 13 of the saliva tests were later confirmed by additional nasopharyngeal tests. The results were reported Friday in the New England Journal of Medicine.”
The early data seem to show that, for early diagnosis, saliva testing might actually be more accurate than nasopharyngeal testing.